Wish to keep on prime of the science and politics driving biotech right now? Enroll to get our biotech publication in your inbox.
Asking once more! Might you inform us what you consider The Readout? Fill out this survey!
Immediately, some attention-grabbing tidbits from STAT’s Breakthrough Summit West in San Francisco, AstraZeneca’s antibody helped forestall Covid-19 in immunocompromised sufferers, and extra.
Google, NVIDIA say AI already impacting drug discovery
Synthetic intelligence is already making its mark in drug discovery, leaders from NVIDIA and Google stated yesterday at STAT’s Breakthrough Summit West. We don’t but have an AI-developed drug. However there have been extra investigational new drug functions to the FDA that cite AI, STAT’s Katherine MacPhail and Mario Aguilar write. And a few early-stage readouts aided by AI could also be exhibiting indicators of success.
“We’ll, hopefully in, name it 10 years or 15 years time, get to that drugs n-of-1. If the method and the manufacturing of medicines can actually, actually be diminished, then we don’t have to fret about these economies of scale of drug manufacturing, we are able to truly construct and manufacture these medication,” an government at NVIDIA stated.
Learn extra.
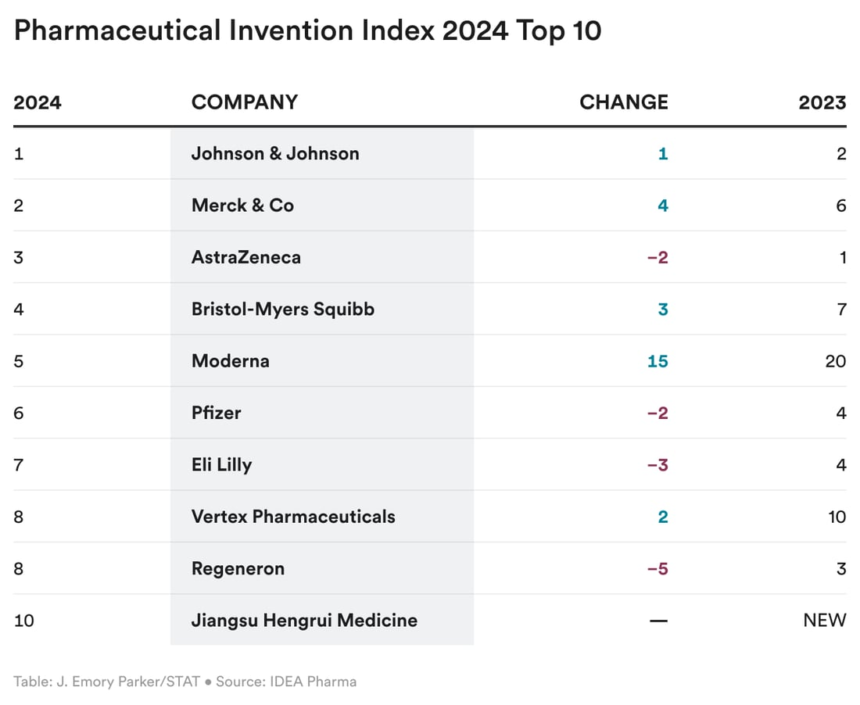
Probably the most progressive and ingenious drugmakers
Which drugmakers are the highest R&D performers — and the way do you parse innovation from invention? The thirteenth Pharmaceutical Innovation and Invention Index, which was launched on the STAT Breakthrough Summit West, makes this distinction in a rating of the biggest pharmaceutical firms.
The businesses flagged as notably ingenious are J&J, Merck, and AstraZeneca — due to their plush pipelines and novel experimental medicines. In the meantime, Novo Nordisk, GlaxoSmithKline, and Pfizer have been deemed glorious at innovation up to now yr, because of their capacity to commercialize new medicines.
Learn extra.
AstraZeneca antibody diminished Covid-19 danger in immunocompromised
From STAT’s Jason Mast: AstraZeneca stated {that a} new long-acting antibody diminished immunocompromised sufferers’ dangers of contracting symptomatic Covid-19 infections in a big Section 3 prevention trial.
That might imply a brand new choice for weak people — comparable to sure most cancers sufferers and transplant recipients — who’ve been awaiting medication that may present extra safety since Evusheld, AstraZeneca’s earlier antibody, was yanked from the market within the face of latest variants in early 2023. One new choice was already licensed final month.
Nonetheless, it’s not clear whether or not the brand new antibody, sipavibart, will truly attain market. SARS-CoV-2 has developed for the reason that firm started creating the drug and sipavibart is just not thought to successfully neutralize variants with a mutation referred to as FL456, which many circulating variants now carry.
The trial had two main endpoints: Discount in illness from any variant, and discount in illness from variants and not using a FL456 mutation. AstraZeneca stated sipavibart met each these endpoints. It has not but stated how sipavibart fares towards variants with FL456 mutations. Regulators will doubtless be eager to know.
Reside! From STAT Breakthrough Summit West
On this week’s “Readout LOUD,” we’re dwell in San Francisco at STAT’s Breakthrough Summit West. AI is an enormous theme this yr, all over the place, so on that be aware, we chat with our AI correspondent — and up to date Pulitzer finalist — Casey Ross, who sat down on stage to focus on AI-centered drug discovery with each NVIDIA and Google-backed Isomorphic Labs.
We additionally focus on this week’s biotech information, together with but extra weight problems drug information, this time from Roche, and the closing of Novartis’ acquisition of MorphoSys.
Hear right here.
Biogen, Ionis genetic drugs fails in ALS trial
An try by Ionis and Biogen to deal with non-genetic types of ALS with an experimental gene-targeting drugs has failed. The businesses examined a drug, BIIB105, in a 99-patient trial, however it failed to indicate enchancment in sufferers — so they’re discontinuing additional research.
Solely about 10% to twenty% of ALS instances are genetic, however some promising preclinical analysis steered that silencing the ATXN2 gene may hep any affected person who develops ALS, STAT’s Jason Mast writes. And whereas the drug did certainly decrease ATXN2 ranges within the spinal fluid, it didn’t appear to gradual the dying of affected person neurons.
Ionis nonetheless has a therapy for FUS-driven ALS, a genetic type of the illness, in Section 3 research. The NIH is funding efforts to make customized medication for the extraordinarily uncommon genetic drivers of ALS.
Learn extra.
Extra reads
- Microsoft’s Peter Lee says ChatGPT shouldn’t be used for preliminary analysis, STAT
- PDS Biotech’s survival profit is absurdly overstated, STAT
- Amgen drug wins U.S. approval for kind of superior lung most cancers, Bloomberg