Topline
Ozempic maker Novo Nordisk on Thursday teased a possible successor to its blockbuster weight reduction drug Wegovy, the newest in a line of next-generation weight problems remedies in growth as shoppers flock to the highly effective medication and opponents together with Eli Lilly, Pfizer and an array of startups shut in to assert a share of the profitable market.
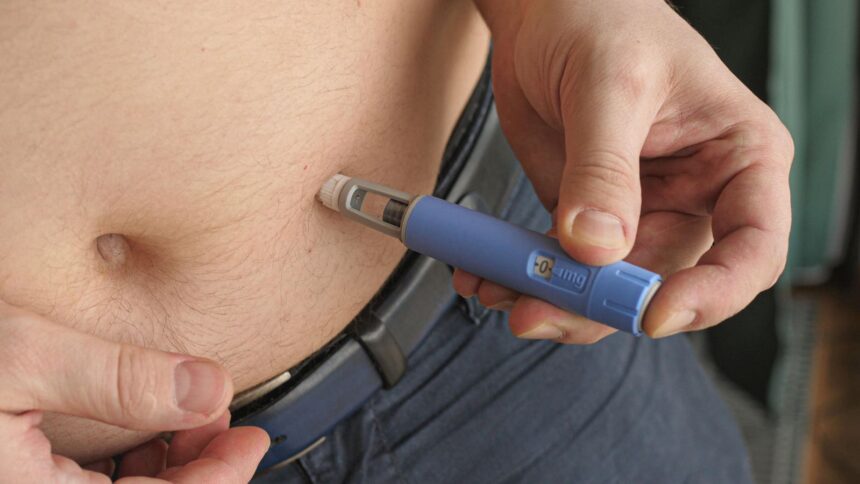
Novo Nordisk’s oral therapy may cast off the necessity for normal injections.
Key Info
At an investor occasion on Thursday, Novo Nordisk shared information from an early stage scientific trial of an experimental weight reduction drug referred to as amycretin, an oral therapy that trial outcomes recommend could possibly be extra highly effective than the corporate’s well-liked weight reduction drug Wegovy.
The capsule, taken as soon as a day, helped sufferers within the trial drop 13% of their weight over 12 weeks, a extra spectacular rate than Wegovy, the place sufferers misplaced round 6% at 12 weeks.
Amycretin successfully combines the performance of two totally different medication right into a single molecule that targets two hormones concerned in regulating starvation and blood sugar ranges: GLP-1 and amylin.
GLP-1 is similar hormone focused by semaglutide and tirzepatide—generic names for Novo and Lilly’s diabetes and weight problems blockbusters Ozempic, Wegovy, Mounjaro and Zepbound—and firms together with Novo and Zealand Pharma view amylin as a one potential goal to construct subsequent technology of weight reduction medication round.
Novo can also be testing a subcutaneous type of amycretin delivered by common injections like Wegovy and Zepbound, although the early stage trial is ongoing and information just isn’t anticipated to be launched till round 2025.
Novo’s head of growth Martin Lange Holst stated the promising trial outcomes justify additional analysis into the capsule and {that a} bigger Part 2 trial would start within the second half of the yr.
When will amycretin be obtainable?
It’s going to nonetheless be a few years earlier than Novo’s amycretin hits pharmacy cabinets. Information from the mid-stage trial won’t be obtainable till round 2026, on the earliest, and regulators are prone to need an much more complete trial to evaluate efficacy and security to authorize it. It’s doable later trials received’t replicate the promising outcomes from the early stage trial—the majority of medication fail throughout scientific testing—or Novo could select to focus its consideration elsewhere. Information from the injectable amycretin is prone to have an effect on Novo’s future growth pathway for the drug. Unwanted side effects and security may even must be monitored in a bigger cohort of individuals to see whether or not the drug makes a viable therapy. Novo stated the security and facet impact profile is according to its different GLP-1 medication, which may embrace a wide range of gastrointestinal points like nausea, vomiting, constipation and diarrhea.
Information Peg
Novo’s inventory hit a brand new excessive after the corporate revealed a few of its future plans on Thursday, gaining 9% and sending its market capitalization hovering to virtually $610 billion. The corporate, which dethroned Bernard Arnault’s LVMH as Europe’s largest final yr, is now extra invaluable than Elon Musk’s Tesla.
Huge Quantity
$100 billion. That’s how a lot some analysts believe the weight problems drug market can be price by the tip of the last decade. Some assume the market is potentially price far more. Novo, alongside Eli Lilly, dominate the market and are prone to proceed doing so for a while.
Additional Studying